Stem cell therapy to treat scars & wounds is relatively early.
We’ve broken down each study in detail, but we know it’s a lot to digest.
At the start of the article, we’ve provided an initial summary of what all the research is telling us.
If you want to look at any study in particular, use the Content Table on the left to go to a particular study.
We hope this is helpful!
Scars Findings
What the Research Says About Stem Cells for Scars
Looking at the latest data, clinical research consistently shows that stem cell therapy is a safe procedure for treating a variety of scars, including those from burns, acne, and surgery. The most common benefit reported across multiple studies is a clear and consistent improvement in the scar’s appearance, texture, and symptoms. Patients have seen better skin color and thickness, and a reduction in issues like itching and tightness. For fresh burn wounds, the therapy has also been shown to significantly speed up healing time.
The research indicates that the cells do not work by turning into new skin. Instead, they work primarily by releasing “healing signals” that calm inflammation, stimulate the body’s natural repair processes, and encourage the healthy remodeling of collagen. The most commonly tested and effective cell source is fat (adipose) tissue, often used as a minimally processed mixture called Stromal Vascular Fraction (SVF). Bone marrow-derived stem cells have also shown strong results.
It is important to note the limitations found in the research. The field is still in its early stages, and most trials have been small and have had short follow-up periods. The studies also use very different methods—including different cell sources, doses that are often not standardized, and various delivery techniques—which makes it difficult to compare results and determine the single best approach just yet.
If you’re looking at Stem Cell Clinics abroad, the biggest risk your taking is going to a clinic following poor standards. That’s why we take our vetting process so seriously. Read more about our process & why we do what we do below
Previous Trials for Stem Cells Treating for Burn Wounds
Allogeneic Bone Marrow Stem Cell Treatment for Burn Wounds: USA
You can read more about the study here.
This trial tested whether donor-derived bone marrow mesenchymal stem cells (BM-MSCs) could safely speed up healing and improve scarring in patients with deep second-degree burn wounds. It was a Phase I dose-escalation clinical trial.
RESULTS SUMMARY
All 10 patients healed completely. People treated with the higher dose healed nearly 3× faster and showed signs of better scar recovery, including improved skin color and even hair regrowth in some cases.
No one had an adverse reaction.
The stem cells seem to work by reducing inflammation and releasing healing signals, not by turning into skin cells themselves.
Study Details:
- Participants: 10 patients aged 18+ with deep second-degree burns (up to 20% of total body surface area)
- Wound areas: Ranged from ~28 cm² to over 820 cm², across arms, legs, chest, and wrists
- Procedure: Split into 2 groups, with 5 patients each, receiving either a low or high stem cell dose
- Delivery method: Topical application (under transparent film dressing), or subcutaneous injection if needed
- Cells used: Allogeneic bone marrow–derived mesenchymal stem cells (BM-MSCs)
Dosage:
- Dose 1 group received 2,500 BM-MSCs per cm²
- This means that for every 1 square centimeter of burn wound, patients in the Dose 1 group were treated with 2,500 bone marrow-derived mesenchymal stem cells
- So if a patient had a 50 cm² wound, they received: 2,500 × 50 = 125,000 stem cells total per treatment.
- Dose 2 group received 5,000 BM-MSCs per cm²
- Patients in the Dose 2 group received double the amount:
- For every 1 cm² of wound, they got 5,000 stem cells.
- So if someone had the same 50 cm² wound, they would receive: 5,000 × 50 = 250,000 stem cells total per treatment.
- Applications: 1 or 2 per patient, depending on healing progress
- Total cells ranged from ~69,750 to 1.33 million per person in Dose 1, and ~157,500 to 8.2 million in Dose 2
Follow-up: Patients monitored for at least 6 months, some followed up to 1 year
How Cells Were Prepared:
- Source: Bone marrow from a young healthy male donor, collected from the posterior iliac crest
Culture method:
- Mononuclear cells were extracted from donor bone marrow using density gradient centrifugation (a method that separates cells based on their weight by spinning them in a liquid). From this layer, mesenchymal stem cells were isolated and expanded in culture.
- Cultured in alpha MEM with fetal bovine serum, penicillin, and streptomycin
- Expanded through 3 passages and cryopreserved
- Vehicle: Stem cells were resuspended in preservative-free saline before application
Purity testing:
- The study did not specify MSC markers (like CD73, CD90, CD105), but confirmed cells were cultured in a cGMP facility and met clinical-grade standards
- Viability & sterility: Not explicitly reported, but cells were prepared under FDA-regulated protocols
Results:
Would Closure:
- Dose 1: On average, wounds in this group got smaller by 3.64 square centimeters each day after treatment.
- Dose 2: In the higher-dose group, wounds shrank by 10.47 square centimeters per day, almost 3 times faster than Dose 1.
- In patients with larger wounds (over 100 cm²), those who got the higher dose of stem cells (Dose 2) healed much faster. This difference was statistically significant ,meaning it probably wasn’t due to chance (p = 0.002).
Scarring (POSAS scores: a tool used by both doctors and patients to rate how bad a scar looks and feels after healing):
- Both groups improved, but Dose 2 patients had better scores in categories like itching, pigmentation, thickness, and irregularity
- One patient regrew hair follicles only in the treated area
- Another showed pigment recovery except in the part where the dressing had blocked treatment
Cytokine analysis:
- Blood tests showed no increase in rejection markers (IFN-γ, TNF-α), and IL-10 (an anti-inflammatory marker) remained normal. This means there were no signs of rejection or harmful inflammation. The treatment was safe
Regeneration signs:
- Treated areas sometimes showed hair regrowth, re-pigmentation and less fibrosis compared to untreated or previously burned areas
- Extracellular vesicles (EVs) were found in the injection fluid at concentrations >10¹¹/mL, and may have contributed to the healing response
Adverse events:
- None reported. No immune reaction or complications in either group
How Cells Worked
- The BM-MSCs helped by sending out anti-inflammatory and regenerative signals, likely through extracellular vesicles that carried growth factors, mRNA, and proteins.
- They did not turn into new skin cells themselves.
What We Don’t Know
- Long-term safety and efficacy beyond 1 year
- Whether more doses or different cell types could enhance outcomes
- If MSCs can prevent pigment loss or scar formation permanently
Conclusion
This trial shows that allogeneic BM-MSCs can be safely applied to burn wounds and may improve healing speed, scar quality and tissue regeneration. Especially at higher doses.
While the sample was small, the results suggest a clear trend toward dose-dependent benefit and warrant larger, controlled studies.
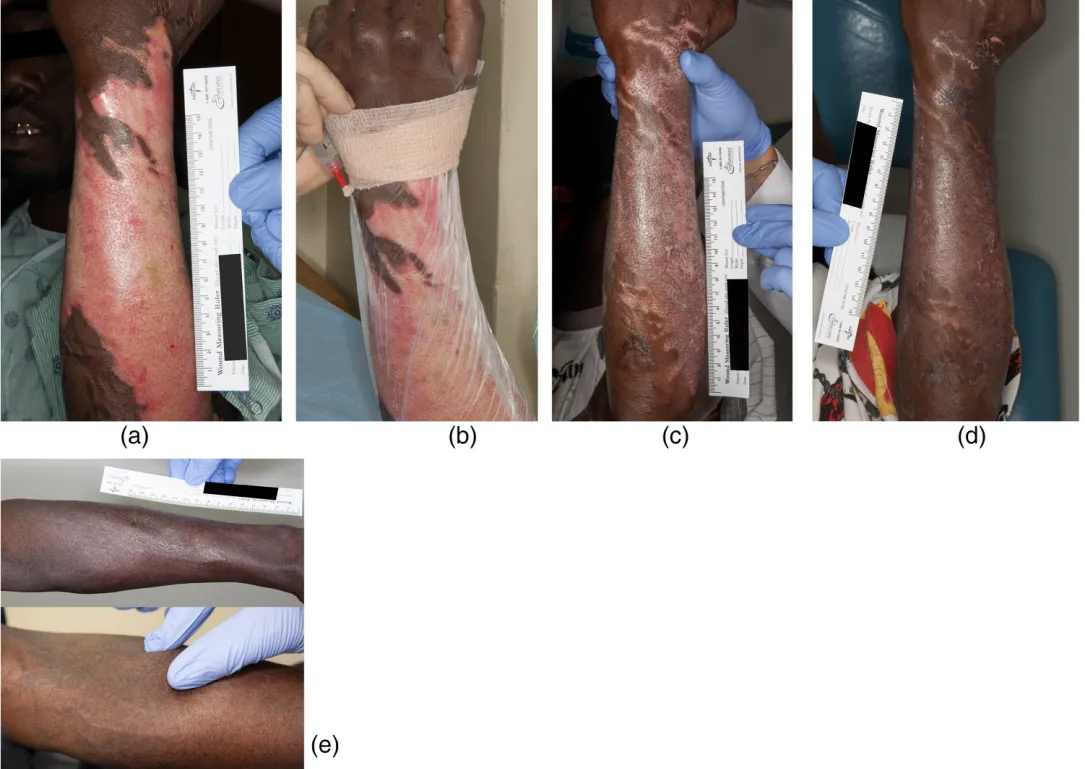
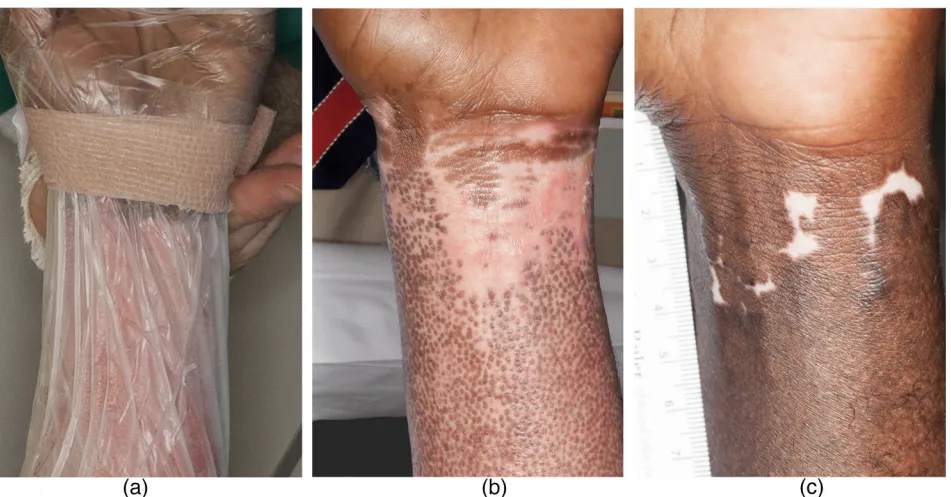
Autologous SVF Stem Cell Treatment + Fractional CO₂ Laser for Burn Scars: Iran
You can read more about the study here.
This clinical trial tested whether injected stromal vascular fraction (SVF), a stem-cell-rich mixture extracted from a person’s own fat, could improve burn scar healing and appearance when used in combination with fractional CO₂ laser therapy. The goal was to compare this combo treatment to laser therapy alone.
It was a double-blind randomized controlled trial, meaning neither the doctors nor the patients knew which scar received which treatment.
RESULTS SUMMARY
All 10 patients saw improvement in both scar areas. However, the area treated with SVF + fractional CO₂ laser had significantly:
• Lower scar severity scores
• Greater improvements in skin thickness and density
• More even skin pigmentation
• Higher patient and physician satisfaction
The combo treatment consistently outperformed laser alone in nearly every measure. No adverse effects were reported.
Study Details
- Participants: 10 patients, aged 25–50 years, with long-standing burn scars (≥3 months old)
- Skin types: Fitzpatrick I–IV (classifies skin from Type I ,very fair, always burns, never tans, to Type VI very dark, never burns, tans deeply based on how it reacts to sun exposure.)
- Scar types: Each patient had sunken, tissue-loss burn scars on at least two different limbs, which allowed researchers to compare two treatments on the same person.
- Design: Each patient had 2 scars treated in 3 sessions:
- One area received fractional CO₂ laser + SVF injection
- The other received fractional CO₂ laser + saline placebo injection
Procedure
Session Schedule (3 total, 1 month apart):
- Session 1: Laser applied to both scars → one scar injected with SVF, the other with saline
- Session 2: Laser only (no injections)
- Session 3: Laser + repeat injections (same scar got SVF again)
Fractional CO₂ Laser Settings:
- Power: 13
- Stack: 2
- Spacing: 800 µm
- Dwell time: 900 microseconds
SVF Cell Preparation & Dose
How cells were prepared:
- 100 cc of fat was removed from each patient’s thigh
- Processed with collagenase (enzyme digestion) to isolate SVF
- Cells were washed, centrifuged, and counted
- Final product was fresh, not cultured (no expansion in lab)
- SVF contained a natural mix of regenerative cells, including adipose-derived stem cells (a type of MSC), endothelial cells, pericytes, fibroblasts, and immune cells, all of which support healing, reduce inflammation, and promote tissue repair
Cell yield & viability:
- 20 million SVF cells per mL
- Out of every 100 cells isolated from the fat, about 81 were still living and potentially able to help with healing. The remaining ~19% were dead or damaged during processing and wouldn’t contribute to the treatment.
- Cell surface markers confirmed using flow cytometry (e.g., CD44, CD90, CD105)
Results
Vancouver Scar Scale (VSS) The VSS is like a scar report card. It helps doctors track how red, thick, stiff, or discolored a scar is over time. The lower the score’s the better
- SVF group:
- Before: 7.50
- After: 4.80
- Improvement: –2.70 (p < 0.001)
- Laser-only group:
- Before: 7.40 ± 1.35
- After: 5.90 ± 1.97
- Improvement: –1.50 (p = 0.007)
- Statistical comparison between groups: The SVF group improved significantly more
Skin Pigmentation (Mexameter – Melanin Index). The Mexameter is a small handheld device that shines light on the skin and measures how much melanin (brown pigment) and erythema (redness) is present, helping doctors track skin color changes in scars or damaged areas over time.
- SVF group:
- Before: 187.57 ± 55.67
The average melanin level in the scar was 187.57, with some variation (±55.67) - After: 202.90 ± 48.75
After treatment, the melanin level increased to 202.90, meaning the skin became darker or closer to the person’s normal pigmentation - Change: +15.33 units (not statistically significant on its own)
- SVF group had +33.69 higher melanin index than control (p = 0.009)
- The melanin improvement in the SVF group alone wasn’t statistically strong, but compared to the laser-only group, the SVF-treated scars had significantly better pigmentation recovery.
- Before: 187.57 ± 55.67
Skin Elasticity (Cutometry Measurements) Cutometry is a test that uses a device called a cutometer to measure how stretchy and elastic your skin is. If the skin bounces back quickly, that’s a sign of healthier, more elastic tissue.
- SVF group:
- R2 (viscoelasticity): No significant change (0.78 → 0.78)
- R5 (pure elasticity): Slight, non-significant increase (0.68 → 0.75)
- R7 (immediate recovery): No significant change (0.54 → 0.51)
- Laser-only group:
- R2: Minor decrease (0.81 → 0.77), not significant
- R5: Slight drop (0.78 → 0.66) — borderline p = 0.057
- R7: Significant drop from 0.59 → 0.48 (p = 0.032)
- While the SVF group’s skin elasticity stayed about the same, the laser-only group’s skin got noticeably stiffer and slower to bounce back. That suggests SVF might protect the skin’s flexibility during scar treatment, even if it didn’t make it dramatically more elastic.
Skin Density & Thickness (Ultrasound Sonography). Ultrasound sonography was used to see how thick and solid the scarred skin was before and after treatment.
A thicker, denser result usually means better healing and stronger, healthier skin.
| Measurement | SVF Group (Before → After) | Laser-Only (Before → After) | Significance |
Complete thickness | 1752.4 → 1120.4 μm | 1695.8 → 1244.6 μm | ↓ in both (SVF: p = 0.061; Laser: p = 0.05) |
| Epidermal thickness | 99.0 → 111.5 μm | 96.1 → 104.8 μm | ↑ in both; SVF group significant (p = 0.016) |
| Dermal thickness | 1653.4 → 1008.9 μm | 1599.7 → 1139.8 μm | ↓ in both; not significant |
| Complete density | 12.01 → 21.50 | 10.89 → 17.27 | Significant ↑ in both (SVF: p = 0.003; Laser: p = 0.018) |
| Dermal density | 9.32 → 17.92 | 7.95 → 14.18 | Significant ↑ in both (SVF: p = 0.002; Laser: p = 0.020) |
| Epidermal density | 52.38 → 52.76 | 51.63 → 50.57 | No significant change |
- The SVF-treated scars became firmer and healthier than the laser-only scars.
- The outer skin layer (epidermis) got thicker, and the deeper layer (dermis) became denser, signs that the skin was rebuilding better.
- Both groups showed some flattening of the scar, but only the SVF group showed significant improvement in skin strength and surface recovery.
Patient & Physician Satisfaction (0–4 Scale). In this study, both the patients and the doctors gave a simple score from 0 to 4 to rate how well they thought the treatment worked.
Group | Patient Score | Physician Score |
| SVF + CO₂ Laser | 3.2 | 3.4 |
| Laser-only | 2.1 | 2.2 |
- All satisfaction scores significantly higher in SVF group (p < 0.01)
What We Don’t Know
- The precise number of cells injected per scar is unknown
- No information on long-term follow-up beyond 2 months
- No biomarker or histological confirmation of how SVF caused changes
- No comparison to other cell types or delivery methods
Conclusion
This small but well-controlled trial shows that autologous SVF injections combined with fractional CO₂ laser appear to significantly improve burn scar healing, pigmentation and patient satisfaction compared to laser alone. Without side effects.
Larger trials with standardized cell dosing and longer follow-up are needed to confirm these promising results and optimize protocols.
Stem Cell Treatment Research for Surgical Scars
Umbilical Cord Stem Cells for C-Section Scars: China
You can read more about the study here.
This clinical trial tested whether applying umbilical cord-derived mesenchymal stem cells (UC-MSCs)t o cesarean section skin incisions could improve scar healing and appearance.
The treatment used a gel made with UC-MSCs, applied on top of the skin after surgery. The goal was to see if these cells could reduce scar thickness, redness, stiffness, and discoloration compared to a placebo gel.
It was a randomized, double-blind, placebo-controlled clinical trial, meaning neither the patients nor the doctors knew who got the real stem cell gel versus the fake one.
RESULTS SUMMARY
All 90 women completed the study and were followed for 6 months. However, no statistically significant difference was found between groups.
That means even though the stem cell gel groups had slightly better scar scores, the difference wasn’t large enough to confidently say it wasn’t due to chance.
Still:
• The high-dose group had the lowest scar severity score
• No side effects were reported
• 90% of women said they would try the treatment again
Study Details:
- Participants: 90 healthy women (ages 21–35) having their first C-section (called primiparous) at full term (37–42 weeks)
- Skin types: Not reported by Fitzpatrick scale, but women with keloids, skin disease, or gel allergies were excluded
- Scar type: Surgical linear scar from cesarean section (lower abdominal skin incision)
Design:
- Each participant was randomly assigned to one of three groups:
- Placebo group: Received a plain hydrogel with no stem cells
- Low-dose group: Received 3 million UC-MSCs mixed into hydrogel
- High-dose group: Received 6 million UC-MSCs in hydrogel
Application: The hydrogel was applied once daily for 6 days directly on the stitched C-section incision.
Procedure
Session Schedule: Daily for 6 days, immediately after surgery:
- Placebo group: 6 days of placebo gel
- Low-dose group: 3 days of UC-MSC gel + 3 days of placebo
- High-dose group: 6 days of UC-MSC gel
- No laser or injection treatments were used. This was a surface gel treatment only.
UC-MSC Cell Preparation & Dose
- Umbilical cord tissue was donated by healthy mothers with consent
- Tissues were digested using enzymes (collagenase and trypsin)
- Cells were filtered, cultured, and expanded under sterile lab conditions
- Cells were mixed with sodium alginate to form a gelatin-like hydrogel
Cell contents:
- Contained mesenchymal stem cells with >95% expressing markers like CD73, CD90, CD105
- Negative for contaminants like viruses, immune cells (CD45, HLA-DR), and bacteria
- Stored frozen at -80°C, with >80% viability after thawing
Doses used:
- 3 million or 6 million cells total per patient, depending on group
- Much lower than many other MSC trials, which often use 100–1000 million cells
Results
Vancouver Scar Scale (VSS)
The VSS is a scoring tool doctors use to grade scars based on:
- Pigmentation (color)
- Vascularity (redness)
- Pliability (stiffness)
- Height (raised vs flat)
Lower scores mean better-looking scars. - VSS scores at 6 months:
- Placebo group: 6.43
- Low-dose group: 5.18
- High-dose group: 4.71
➡ Improvement trend seen with stem cells, but not statistically significant (p > 0.05) - Although the high-dose stem cell group showed the lowest scar severity score, the differences were not statistically significant. Meaning the improvements could have occurred by chance rather than because of the treatment.
Skin Pigmentation and Redness
- Pigmentation and erythema around the scar were measured with visual grading tools.
➡ No significant difference between groups.
Skin Elasticity and Thickness
- Not measured directly with tools like cutometers or ultrasound.
➡ Only subjective scar thickness scores via VSS were tracked.
Bloodwork and Safety Monitoring
Tests were done before and after for:
- Liver and kidney function (ALT, AST, BUN, uric acid)
- Tumor markers (CA125, CA153, CA199, CEA)
➡ No abnormalities found in any group.
Patient & Physician Satisfaction (5-point scale)
- Patients rated their satisfaction as:
- None” – 49%
- “Slight” – 51%
- “Moderate” to “Very Good” – 0%
- However, 90% said they would use the treatment again and would recommend it to a friend.
What We Don’t Know
- The low dose (3–6 million cells) may have been insufficient. Many studies use 100M+ cells
- Scar healing takes 12–24 months, but this study only followed women for 6 months
- Patients with keloids or difficult scars were excluded, who might have benefitted more
- No detailed skin elasticity or imaging tools were used to confirm tissue remodeling
- The study used donor (allogeneic) cells. We don’t know if results would differ with autologous (self) cells
Conclusion
This well-controlled trial shows that umbilical cord stem cells applied as a hydrogel after C-section are safe and easy to use.
However, no clear scar improvement was seen at the tested doses after 6 months.
Larger trials with higher cell counts, more advanced scar imaging, and longer follow-up are needed to confirm whether UC-MSCs can meaningfully reduce surgical scarring.
Previous Trials for Stem Cells treating Acne Scars and Atrophic Scars
2025 Review on Adipose-Derived Stem Cells (ADSCs) for Treating Scars: Australia & Iran
You can read more about the review here.
A team of researchers from Australia and the Iran came together to examine whether fat-derived stem cells actually work in treating pathological scars. Including keloids, hypertrophic scars and atrophic scars like acne or injury marks.
The team included dermatologists, stem cell researchers, and plastic surgeons, and their review was published in Stem Cell Reviews and Reports in 2024.
RESULTS SUMMARY
Stem cell therapy using fat-derived cells, especially SVF (Stromal Vascular Fraction), consistently improved scar appearance, depth, texture, and symptoms like itching and tightness in all clinical trials reviewed.
In particular, it showed clear clinical success in treating atrophic scars (like acne or surgical scars), and even outperformed steroid injections for keloid scars in one study.
These cells work not by turning into new skin, but by releasing healing signals that reduce inflammation, support tissue repair, and stimulate collagen remodeling.
No serious side effects were reported in any clinical trial.
What they looked at
9 clinical trials that used Adipose-Derived Stem Cell (ADSC) therapies to treat scars in real patients.
Scars treated included:
- Atrophic scars (e.g. acne scars, surgical scars)
- Keloid scars (overgrown, raised scars)
- Hypertrophic scars (thick but within wound margins)
Most studies used a point-of-care fat extract called SVF rather than lab-cultured stem cells.
What They Were Trying to Find Out
- Is SVF or ADSC therapy effective at improving scars. Texture, color, depth and symptoms like itching?
- Does combining SVF with other treatments (like lasers or PRP) improve results?
- How safe is this treatment when used in humans?
- Are outcomes consistent even without knowing the exact cell composition?
What is SVF?
- Stromal Vascular Fraction (SVF) is a stem cell–rich mixture extracted from your own fat (usually through a small liposuction). It contains:
- Adipose-derived stem cells (ADSCs)
- Blood vessel cells
- Immune cells (like macrophages)
- Growth factors
- It’s processed on the same day and injected directly into the scar. No lab expansion, no culturing, no waiting.
- Think of it as a natural healing concentrate made from your own fat.
What They Found
- Effectiveness
- Across all 9 clinical trials, SVF and other fat-derived products improved scar quality, especially in:
- Atrophic scars (6 studies)
- Keloid scars (1 study)
- Hypertrophic scars (2 studies)
- For Atrophic Scars:
- SVF injections helped restore skin structure, improving collagen density, hydration, epidermal thickness, and overall appearance.
- In one study, SVF worked just as well as fractional CO₂ laser for acne scars.
- Patient satisfaction was high, and results were noticeable after 1–3 sessions.
- For Keloids:
- SVF injections outperformed triamcinolone, a common steroid, in reducing itch, thickness, and improving skin texture.
- For Hypertrophic Scars:
- SVF combined with laser therapy reduced itch, size, and discoloration better than laser + steroid.
- Across the board, SVF improved both the way scars look and how they feel, including texture, flexibility, and itch relief.
- Across all 9 clinical trials, SVF and other fat-derived products improved scar quality, especially in:
- Mechanism of Action
- The healing wasn’t due to the stem cells turning into new skin.
- Instead, SVF works by sending out healing signals, anti-inflammatory molecules, growth factors, and cytokines that stimulate your body’s natural repair process.
- This is called paracrine signaling, and it’s likely the main reason SVF is effective.
- Dosing & Quality Control
- None of the clinical trials measured how many cells were injected.
- Doses were based on volume (e.g. 1 mL per scar area), not cell count.
- No studies tested for cell surface markers (like CD90 or CD105), and composition likely varied between patients.
- This means the treatment works, but we don’t know exactly what’s in each dose.
- None of the clinical trials measured how many cells were injected.
- Safety
- All clinical trials reported no serious side effects from SVF or ADSC treatments.
- Minor issues (like temporary redness or swelling) resolved quickly.
- Since the stem cells came from each patient’s own body (autologous), immune rejection was not a problem.
- Limitations Across Trials
- Small sample sizes (most trials had fewer than 50 patients).
- No standardization of how SVF was processed or injected.
- Lack of blinding in some studies.
- Short follow-up periods (many ≤ 6 months).
- No consistent way to measure scar improvement across studies.
- Small sample sizes (most trials had fewer than 50 patients).
What They Concluded
Stem cell therapy using SVF from fat is:
- Safe
- Well-tolerated
- Clinically effective at improving a range of scars, especially atrophic scars like acne or trauma scars.
The healing effects come from the signals stem cells send, not from them becoming new skin.
But we still need:
- Standardized methods for how SVF is prepared and used
- Better outcome tracking tools (e.g., consistent scales, longer follow-ups)
- Trials with known cell doses and biomarker testing
- More data on how it compares to other scar treatments over time
Previous Research looking at Stem Cells treating Keloids
Phase 1 Fat-Derived Stem Cells (SVF) for Keloid Scars: Uganda
You can read more about the study here.
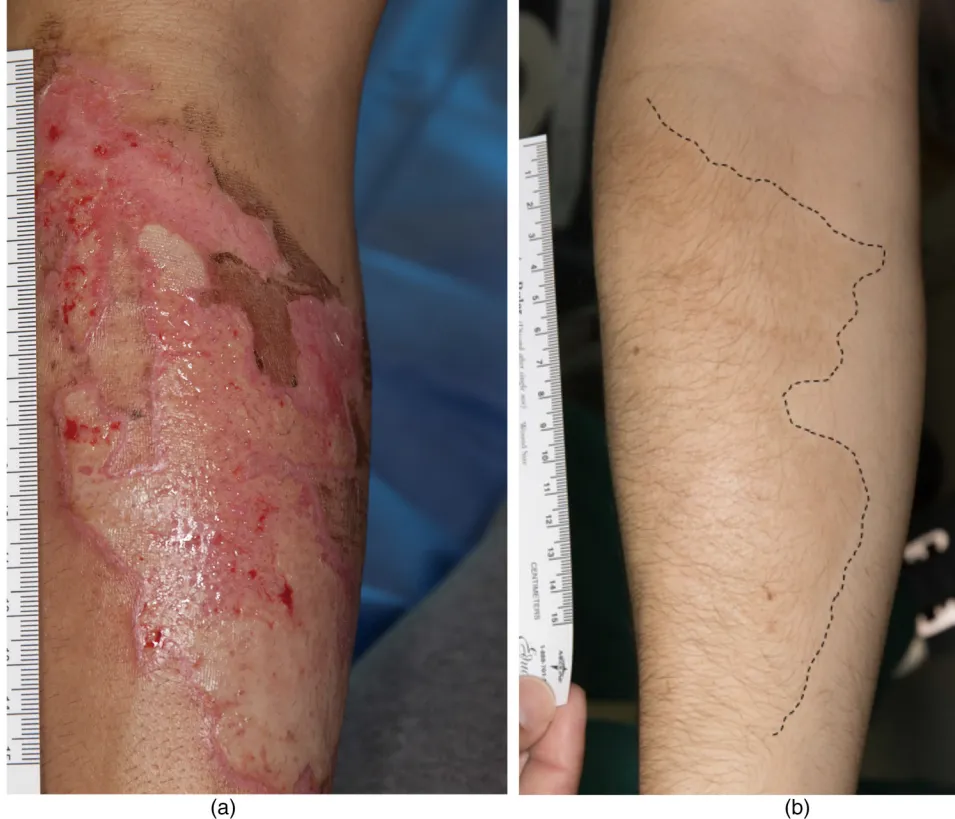
This clinical trial tested whether injecting a patient’s own fat-derived stromal vascular fraction (SVF) could safely treat keloids. A type of raised, painful, and often itchy scar that grows beyond the edges of a wound.
The treatment involved harvesting fat tissue through liposuction, isolating the stromal vascular fraction (which contains stem cells and other regenerative cells), and injecting it directly into the keloid. The goal was to see if this method was safe, tolerable, and realistic to use in low-resource settings.
This was a randomized controlled pilot trial, meaning patients were randomly assigned to receive either SVF or the standard steroid injection (Triamcinolone Acetonide, or TAC).
The study was conducted by researchers from Makerere University College of Health Sciences and Kirruddu National Referral Hospital in Uganda. Led by Dr. Ronald Mbiine and a multidisciplinary team of surgeons, immunologists, and public health specialists.
RESULTS SUMMARY
The primary goal of the study was to see if injecting fat-derived stromal vascular fraction (SVF) into keloids is safe and it was. No side effects were reported in the SVF group.
The secondary outcome was whether SVF helped improve scar healing. Both the SVF and steroid groups saw scar improvement, but the SVF group showed better results at 1 month. By 3 months, results were similar in both groups.
SVF works by delivering stem cells and other regenerative cells that reduce inflammation, slow down scar-forming signals, and improve tissue repair.
The only adverse event in the study was a mild skin ulcer in the steroid group, which healed without complications. No complications occurred in the SVF group.
Study Details
Participants:
- 8 adults aged 20–39 with visible keloids
- 4 patients received SVF
- 4 received steroid injections (TAC)
- All keloids were small (≤4 cm³), and participants had not received other keloid treatments in the last 3 months.
Scar type:
- Raised, fibrotic scars (keloids) from various causes (e.g., piercings, cuts, acne).
- Mostly found on the chest, earlobe, and abdomen.
Design:
- Each participant was randomly assigned to one of two treatment groups:
- TAC Group (Control):
- Received 10 mg/mL Triamcinolone Acetonide injected into the keloid.
- Standard treatment commonly used in low-income countries.
- SVF Group (Experimental):
- Underwent liposuction to extract fat from the outer thigh.
- Fat was processed to isolate stromal vascular fraction (SVF), including:
- Mesenchymal stem cells
- Pericytes
- Nucleated immune cells
- SVF was injected into the keloid at a 1:1 volume ratio based on scar size.
- TAC Group (Control):
Procedure Timeline
- Day 0: Treatment administered (SVF or TAC)
- Follow-ups: At Day 1, Week 1, Month 1, and Month 3
- Assessments included:
- Adverse events (using CTCAE v5.0 criteria)
- Scar appearance using Patient and Observer Scar Assessment Scale (POSAS)
- Keloid thickness (measured via ultrasound)
SVF Cell Preparation & Dose
- Fat harvested under local anesthesia using tumescent liposuction.
- SVF was extracted by breaking down the fat with enzymes and then spinning it in a Centrifuge to collect the healing cells.
- Final cell dose averaged 2.7 million viable cells, injected directly into keloid tissue.
- Cells were counted using a Neubauer chamber and Trypan Blue staining.
- Viability of the cell prep: 94% on average
Results
- Safety:
- No complications in the SVF group.
- 1 patient in the TAC group developed a minor ulcer at the injection site (resolved without surgery).
- Feasibility:
- All procedures completed successfully.
- SVF prep and injection took ~5 hours; TAC took ~1 hour.
- All participants returned for follow-ups, and measurements were consistently reproducible.
- Scar Appearance (POSAS Score):POSAS scores improved in both groups.
- At 1 month:
- SVF: 14.8-point improvement
- TAC: 10.3-point improvement
- At 3 months:
- SVF: 7.8-point improvement
- TAC: 7.5-point improvement
- Symptom relief (itching, stiffness) was reported by all patients in both groups.
- At 1 month:
How the SVF Treatment Works
- According to the researchers, stromal vascular fraction improves scars by:
- Reducing inflammation
- Inhibiting excess collagen production
- Suppressing fibrotic signaling pathways, including:
- TGF-β (Transforming Growth Factor Beta)
- p38/MAPK pathway
- This leads to less dense, more flexible scar tissue and faster healing.
- Reducing inflammation
What We Don’t Know (Yet)
- This was a small pilot study, not powered to measure long-term effectiveness.
- The study only included 8 people, so larger trials are needed to confirm results.
- All SVF participants were women (due to small randomization sample).
- Only short-term scar outcomes were measured (3-month follow-up).
- Cost and scalability in larger populations were not evaluated.
Conclusion
This early-stage trial shows that injecting autologous fat-derived stromal vascular fraction (SVF) into keloids is safe, well tolerated and feasible in low-resource settings like Uganda. It may offer an alternative to steroid injections, with fewer side effects and promising early results.
Conclusion: Stem Cell Research for Treating Scars
Overall, there hasn’t been tons of research in this area.
The most common stem cell type studied for treating scars & wounds by far is Stromal Vascular Fraction (SVF), which is a stem-cell-rich extract from adipose (fat) tissue.
How it Works
Across all the research, stem cells primarily worked through paracrine signaling, not differentiation.
None of the studies reported that stem cells transformed into keratinocytes or skin cells or integrated long-term into skin structures.
Are there positive Results
Across five clinical studies involving over 130 patients, stem cell treatments using SVF and bone marrow-derived MSCs delivered clear, consistent improvements in scar healing, appearance and patient satisfaction. All without serious side effects.
The one outlier? A trial using a low-dose umbilical cord stem cell gel that didn’t show strong results, likely because the dose and delivery just weren’t enough to make a difference.
Remember, if you are looking into getting Stem Cells for your own scars or wounds, these trials are super early, so success in your treatment isn’t guaranteed.
Just because these trials had positive results, doesn’t mean Clinics will deliver the same results. They may not have the same preparation methods, quality checks or expertise. So be careful!
If you’re deciding which country is best for you, or want to talk about clinics we’ve already vetted, fill out our form below. Our team will guide you with clear, honest answers.
Alt Treatment is a free, independent platform that helps you understand stem cell therapy & decide if it’s right for you.
We break down complex information into clear, honest guidance. When you’re ready, we can connect you with verified clinics that meet your needs, in the right location & often with exclusive discounts.
There’s no charge to use our platform. No hidden fees. No pressure. Our main aim is to genuinely help you figure out if treatment is right & the best places to consider.
If you want to talk, fill out our form here & our personal concierge team will reach out.
Most stem cell therapies work by releasing healing signals that reduce inflammation, regulate the immune system, and stimulate the body’s own repair processes. To learn more, see our full guide on How Stem Cell Treatments Work
Common side effects of getting Stem Cells are fever and local pain/swelling at the injection site. We go into all of the side effects in more detail in our Stem Cell Therapy Side Effects Article
A lot of countries offer Stem Cell Treatments to some levels for example you can get some treatments in the US & UK but they will be limited.
Other countries include the likes of Japan, South Korea, Colombia & Costa Rica.
To compare countries, our guide on Best Countries for Stem Cell Treatments will be useful.
Fill in your details below
For a discounted offer for Stem Cell Therapy!
