Stem Cell treatment can potentially help with lower back pain & spinal issues, but it’s not always straight forward.
We break down how stem cells can help with different back conditions like lower back pain, sciatica, degenerative discs & look at costs for treatment in different countries.
Our teams here to help you. Our team has personally vetted Stem Cell clinics globally to make sure you can choose clinics you can trust. We continually speak to every patient who gets treatment with our clinics, so we can track success rates but also clinic quality continuously.
How Do Stem Cell Treatments Help with Back Pain?
Stem cell therapy for back pain works by calming inflammation, repairing damaged discs and joints, & encouraging the body to heal itself naturally.
In theory, these cells can turn into new disc or cartilage tissue. But so far, most MRI scans haven’t shown that happening. But it could also be not many studies have looked for it closely, so we just don’t know yet. To break it down further:
How Stem Cells Help

How Stem Cells Can Help Reduce Inflammation in Back Pain
Anti-Inflammatory Signals
Source: AltTreatment.io
To learn more about the full process, check out this article explaining how Stem Cell Therapy works in detail.
Stem Cells Used to Treat Back Pain
In clinics, doctors are using Mesenchymal Stem Cells derived from either your own bone marrow or fat tissue OR coming from Umbilical cord tissue (also known as Wharton’s Jelly) to treat back & spinal issues.
In research trials, teams are also exploring Neural Stem cells, IPSC cells, Embryonic cells and more
What Types of Back Pain Can Stem Cell Therapy Treat?
Stem cell therapy may help treat chronic lower back pain, herniated discs, degenerative disc disease, spinal arthritis, sciatica, and even some spinal cord injuries by reducing inflammation and repairing damaged tissues. Here’s a quick look at the types of back pain it may help:
Degenerative Disc Disease Stem Cell Treatments
Think of your spinal discs like shock absorbers. Over time, they can wear out, causing pain and stiffness. Stem cells step in to regenerate these damaged discs, potentially improving both comfort and mobility.
Herniated Discs Stem Cell Treatments
A herniated disc can press on your nerves, leading to sharp pain and even numbness. Stem cell therapy aims to reduce inflammation and repair the damaged disc tissue, offering relief without the need for invasive surgery.
Spinal Arthritis (Facet Joint Pain) Stem Cell Treatments
If arthritis in your spine makes every movement feel stiff and painful, stem cells can help calm the inflammation and improve joint health, giving you more freedom to move.
Sciatica Stem Cell Treatments
That shooting pain down your leg? Stem cells could help. By targeting the inflamed or compressed sciatic nerve, they may ease discomfort and encourage natural healing.
Can Stem Cells treat Chronic Lower Back Pain?
Yes, stem cell therapy may help with chronic lower back pain by targeting damaged spinal discs, inflammation, and slow-healing injuries. Unlike steroid injections, which only provide temporary relief, stem cells aim to repair tissue and reduce inflammation at the source.
Spinal Cord Injuries Stem Cell Treatments
While still experimental, early research shows promise for using stem cells to repair nerve damage and potentially restore function in cases of spinal cord injury.
Treatment Process for Stem Cell Therapy on your back
This is the process can expect if you get Stem Cell Treatment on your back.
- Stem Cell Collection:
- If the stem cells are coming from your own body, they’re taken from either your bone marrow (usually from your hip) or fat tissue through a quick, minimally invasive procedure.
- If donor cells are used, typically from umbilical cord tissue, they’re sourced from a certified lab, ready to go.
- Processing the Stem Cells:
- If you’re using your own cells and getting a high dosage treatment, the mesenchymal stem cells are isolated & are grown in a lab for 4 to 6 weeks.
- If you’re using your own cells and getting a same day procedure, there’s no processing involved.
- If you’re using umbilical cord derived MSC Cells, the clinic will already have the cells cultured before you arrive.
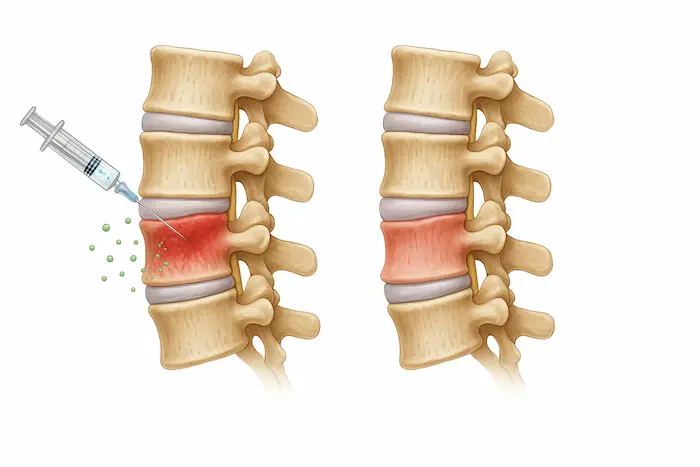
- Depending on your condition, the doctor will usually apply one of the following injections:
- Intradiscal Injection – Directly into a damaged spinal disc to repair tissue.
- Facet Joint Injection – Into the small joints along the spine to reduce pain and inflammation.
- Epidural Injection – Near the spinal nerves, similar to a steroid injection.
- Paraspinal Muscle Injection – Into spinal muscles for overall regeneration (less common).
- IV Treatment: If clinics can’t do any of the above injections, they may give you an IV treatment instead.
Stem Cell Therapy for Back Pain Recovery Time
Recovery time for Stem Cell Treatment on your back takes 2-6 weeks to let the stem cells work. You’ll be able to walk around pretty much straight away, but you should avoid high impact activities.
Get an estimated treatment quote for your specific knee condition
We know getting treatment quotes isn’t easy. Get a personalized, estimated quote for any country so you can compare options for your knee’s globally.
Get Free Cost Estimate
How Much Does Stem Cell Therapy for Back Pain Cost globally in 2026
Stem Cell Therapy for Back pain can range from $8,000 up to $20,000+ in 2026. This table below goes into more detail
| Country | Cost Range ($ USD) | Cost Guide |
| Colombia | $15,000 | Colombia Cost Guide |
| Thailand | $8,000 to $10,000 | Thailand Cost Guide |
| Panama | $15,000 to $20,000+ | Panama Cost Guide |
Factors that affect cost are:
- Delivery Method. Intrathecal injection for example will be more expensive than an IV treatment.
- How many injections are needed? Depending on the severity & location of your condition, you may need multiple injections in the same treatment.
- Dosage.
- Type of Back Condition you have
We’ve gathered our data in a few different ways & we’re always updating it, so bear with us as we collate more cost information in different countries.
To look at average costs of Stem Cell Therapy in different regions, our article on Stem Cell Therapy costs in different countries might be useful!
Not sure where to get stem cell treatment for your back issue? We’ll help you choose safely & help secure exclusive discounts.
What are the risks of getting Stem Cell Treatments for Back Pain?
Risks of getting Stem Cell Therapy on your back include limited long term data, possible nerve irritation from injections & the need for additional treatments for complex injuries.
- Limited Long-Term Data
- Most back pain and spinal cord studies follow patients for only a short time. Early results look encouraging, but we still don’t know how long the benefits last or whether repeated treatments are needed.
- Varied Results
- People respond differently. Improvements in pain or movement depend on factors like overall health, age & how severe the disc or spinal cord damage is.
- Infection Risk
- Any procedure involving injections carries a small risk of infection. This applies to disc injections for back pain and to spinal procedures used in some spinal cord injury studies.
- Temporary Pain or Swelling
- Mild pain, swelling, or stiffness near the injection site can happen after back-pain treatments. These effects are typically short-lived and similar to what you’d expect after other spine injections.
- No Proven Disc or Nerve Regeneration
- Across all reviewed studies, no human trial has demonstrated clear disc repair or nerve regrowth on imaging. Improvements tend to come from reduced inflammation and better nerve protection, not from rebuilding damaged tissue.
- Nerve Sensations
- With people who have had multiple stem cell injections into their back, we have heard of some having strange nerve sensations. Whilst extremely rare, they did subside.
Stem Cell Therapy vs. Traditional Back Pain Treatments

| Treatment | Cost | Effectiveness | Longevity | Risks |
|---|---|---|---|---|
| Stem Cell Therapy | $8,000–$20,000+ | Promising for reducing inflammation and repairing tissue | Potentially 1–5 years, more research needed | Experimental. Results are varied from patient to patient |
| Cortisone Injections | $1,000–2,500 per shot | Effective for short-term pain relief | A few months | Can weaken cartilage if overused |
| Physical Therapy | $50–$200 per session | Gradual improvement in mobility and strength | Varies by condition | Minimal risk; requires consistent effort |
| PRP (Platelet-Rich Plasma) | $2,000–$5,000 | May improve healing and reduce pain | 6–12 months | Mild swelling or discomfort post-procedure |
Latest research on Stem Cell Therapy for Back Pain
The research into stem cell therapy & Stem cell products for back pain is still evolving, but here are some highlights:
BRTX-100 Stem Cell Therapy for Chronic Lower Back Pain
(To read more about the study yourself, here’s the press release they released on May 13th 2025!)
Who’s Running This Study?
BioRestorative Therapies, Inc. is conducting this Phase 2 clinical trial to test BRTX-100. A stem cell treatment aimed at people with chronic lumbar disc disease (cLDD), a common cause of long-term lower back pain. What makes it unique? BRTX-100 uses a patient’s own bone marrow stem cells, but they’re specially grown in low-oxygen (hypoxic) conditions, which may help them survive better and repair damaged tissue more effectively.
Who Took Part?
The early data includes 15 participants (but the full trial will include up to 99). To join, patients had to have:
- Lower back pain due to lumbar disc degeneration.
- Persistent symptoms despite trying other treatments like physical therapy or pain medications.
How Did It Work?
Each patient received a single injection of 40 million (40×10⁶) mesenchymal stem cells from their own bone marrow, given directly into the damaged disc. Before the injection, the stem cells were blended with something called platelet lysate. A mix of healing proteins taken from the patient’s own blood. This gives the cells an extra boost, helping them survive longer, send better signals to reduce inflammation, and kickstart the body’s natural repair process once they’re inside the damaged disc. Together, this combo may lead to better long-term relief and help some patients avoid surgery altogether. This is a randomized, double-blind study, meaning neither patients nor doctors know who got the real treatment vs. the placebo.
Did the Stem Cells actually rebuild the disc?
BioRestorative didn’t track whether the stem cells turned into new disc tissue. There were no cell labels, no biopsies, and no imaging results showing new disc formation. That means there’s no direct proof the cells differentiated (transformed) into disc cells. But what we do have is growing evidence that the treatment helped reduce pain and improve function over time without serious side effects. Given that, and the fact the stem cells were specially prepared to survive longer and release healing proteins, it’s far more likely that they worked by sending out regenerative signals (not by becoming new tissue themselves). In simple terms: the cells acted more like coaches giving instructions, not turning into new cells themselves.
Results So Far
Safe So Far: No serious side effects have been reported up to 2 years post-injection.
Pain & Disability Improvements: A growing number of patients showed major improvements over time. Here’s the percentage of patients who saw over 50% pain and disability reduction at different checkpoints:
- Week 2: 0%
- Week 12: 13%
- Week 26: 46%
- Week 52: 70%
- Week 104: 67%
What This Means
These early results are super encouraging. BRTX-100 may offer a non-surgical, long-term solution for people struggling with chronic back pain due to disc problems. The fact that pain and disability improvements increased over time suggests real regenerative benefits. Because the treatment uses your own cells, there’s no risk of immune rejection.
Next Steps
The trial is still ongoing and expanding to include more participants. If future data stays positive, BRTX-100 could become a new gold standard for treating lower back disc issues, especially for people trying to avoid surgery.
CELZ-201-DDT Stem Cell Therapy for Chronic Lower Back Pain
(To read more about the study yourself, here’s the press release & here’s the study page)
Who’s Running This Study?
Creative Medical Technology Holdings, Inc. (CELZ) is conducting this Phase 1/2a clinical trial to evaluate the safety, tolerability, and effectiveness of CELZ-201-DDT, an allogeneic (donor-derived) stem cell therapy for patients suffering from degenerative disc disease and chronic lower back pain. The just announced results in January 2025! The trial uses umbilical cord-derived mesenchymal stem cells (UC-MSCs). But here’s the catch, Creative Medical has modified these cells using their own process to enhance their anti-inflammatory and regenerative properties for treating chronic lower back pain.
Who Took Part?
10 participants with chronic lower back pain caused by degenerative disc disease. Patients were required to have persistent pain for at least 6 months and have tried other treatments (e.g., painkillers, physiotherapy, or injections) without success.
How Did It Work?
Participants were randomly divided into three dose groups plus a placebo group. Each patient received six intramuscular injections (three injections per side of the lumbar paraspinal muscles) under ultrasound guidance.
- Low-dose group: Received 6 million (6×10⁶) stem cells.
- Medium-dose group: Received 30 million (30×10⁶) stem cells.
- High-dose group: Received 60 million (60×10⁶) stem cells.
- Placebo group: Received an injection without stem cells.
Results So Far
✅ No major safety concerns – The therapy was well tolerated, with no serious adverse effects reported in the first cohort.
✅ Pain Reduction – Early data suggests that patients receiving CELZ-201-DDT experienced decreased pain levels.
✅ Improved Mobility – The treatment showed potential for enhancing physical function and reducing disability scores in patients.
What This Means
Early results are promising, but more data is needed to confirm long-term effectiveness. CELZ-201-DDT could provide a non-surgical, regenerative alternative for chronic back pain sufferers. Higher doses may be more effective, but further trials are needed to determine the optimal dosage. So far, there’s no evidence that the stem cells actually turned into new tissue. Instead, it looks like they worked by sending out healing signals. Reducing inflammation, calming irritated nerves, and helping the body feel better naturally.
Next Steps
The trial is ongoing and continuing enrollment toward its target of 30 participants. Each patient will be followed for 12 months to evaluate long-term safety, pain relief, and functional improvements.
Umbilical Cord Stem Cell Product Trial for Chronic Lower Back Pain
(To read more about the study yourself, here’s the source!)
Who’s Running This Study?
A South Korean biotech company, CHABiotech CO., Ltd, is behind this research. They specialize in stem cell-based treatments and are testing whether their product, CordSTEM-DD, can help with chronic lower back pain caused by degenerating spinal discs.
Who Took Part?
36 adults (ages 19–69) dealing with serious lower back pain for at least 6 months. They had already tried other treatments (like painkillers, physiotherapy, or injections) without success.
How Did It Work?
Patients were randomly placed into three groups:
- Low-dose group: Got 7 million umbilical cord stem cells (0.7 × 10⁷).
- High-dose group: Got 21 million umbilical cord stem cells (2.1 × 10⁷).
- Placebo group: Got an injection with hyaluronic acid (HA) and saline, but no stem cells.
So, is this the same as traditional stem cell therapy?
Nope. This is a pre-made, lab-grown stem cell product (CordSTEM-DD), not a personalized treatment using the patient’s own stem cells. Think of it like an “off-the-shelf” stem cell treatment—standardized and ready to go, rather than a custom-made procedure where stem cells are taken from a patient and reinjected.
Why Haven’t We Seen the Results Yet?
The study officially ended in April 2023, so why no results? The company submitted them in October 2023, but ClinicalTrials.gov sent them back for revisions in April 2024 (probably to fix missing details or clarify data). They resubmitted in April 2024, and the next review cycle is set for August 2024. Delays like this aren’t unusual—clinical trial data needs to be rock-solid before it’s published. Hopefully, we’ll know soon whether this stem cell product is the real deal for back pain!
A 2023 Review on Stem Cells for Back Pain
A 2023 review covering 14 Studies in the British Medical Bulletin explored how mesenchymal stem cells (MSCs) could help treat back pain caused by worn-down spinal discs. The review concluded that MSCs likely help through signaling, not by becoming new disc cells. While some studies showed MRI improvements like better hydration, the authors found “no conclusive evidence that these reflected MSC differentiation or structural regeneration.” The main benefits appear to come from anti-inflammatory effects and tissue repair signals, not true disc regeneration.
The review also pointed out a few challenges:
- Mixed Results: Not everyone gets the same level of relief.
- Safety Concerns: Rare risks, like infection, need careful monitoring.
- Limited Long-Term Data: More studies are needed to see how long the benefits last.
- Most studies did not clearly report how the stem cells were grown. Details like oxygen levels, use of growth factors, or what was added to the cell culture (like serum) were often missing.
The takeaway from this particular review? Stem cell therapy is promising for back pain but still experimental and needs more research.
Want to dive deeper into the studies? Check out our detailed article on all the research.
What is the success rate of stem cell therapy for back pain?
Stem cell therapy for back pain shows mixed results. Some studies suggest around 40-53% of patients feel pain relief, and up to 74% see better movement. But these studies weren’t the best, we’ll break it down below.
A closer look at less than 12 studies (you can read more about them on this article) on MSC therapy for lower back pain found:
- Pain Relief
– About 53.5% of patients reported noticeable pain relief within 6 months.
– If missing data was assumed to be treatment failure, the worst-case success rate dropped to 40.7%. - Improved Movement & Function
– Around 74.3% of patients saw at least 30% better mobility, meaning they could move more easily and handle daily tasks with less discomfort
– If we assume missing data meant no improvement, that number dropped to 44.1%.
A 2023 review looked at studies on mesenchymal stem cells (MSCs) for lower back pain and found that many patients saw real improvements in pain and mobility. Another review suggests stem cells might help repair worn down spinal discs and reduce inflammation. Potentially a game changer for people who haven’t had success with traditional treatments.
You can read both full reviews here and here.
What Does This Actually Mean?
- Some patients see real relief, but it’s not a guaranteed fix for everyone.
- There’s no universal success rate because results depend on the patient, the clinic, the type of stem cells used and cell count.
- Long-term data is still lacking. Most studies only track results for a year or less
Top Stem Cell Clinics for Back Injuries


Where can I get Stem Cell Treatment for my Back Pain?
From Alt Treatment’s Vetted Clinics! You can get safe Back & Spine treatments in most countries, it’s more dependent on the actual clinic you’re choosing. But there are factors to take into consideration:
Regulations
Different countries have more or less regulations. Japan & South Korea, for example, have legalized cultured stem cell treatments.
Cell Type
Do you want umbilical cord derived cells or your own cells? In the US for example, you can get umbilical cord derived MSCs in Florida.
Medical Team
Are the team actual experts in treating orthopedic conditions? This is a key question to ask.
Cell Quality
Is the clinic working with the right cell processing centres and adhering to GMP standards?
We know it’s a lot to take on board, that’s why our teams are here to help.
So, Does Stem Cell Therapy Work for Back Pain?
For some who have had Stem Cell Treatment for back pain, they’ve seen real differences. But for others, it hasn’t had the impact they wanted.
In terms of research, trials are now progressing & from a treatment perspective, back & spinal issue stem cee treatments are becoming more common.
But, you do need to be careful. Back Stem Cell treatments are more complex than a localized knee injection or an Anti Aging treatment for example.
The clinic you choose plays a huge part in how successful your treatment is. Our team has personally vetted Stem Cell clinics globally to make sure you can choose clinics you can trust.
We continually speak to every patient who gets treatment with our clinics, so we can track success rates but also clinic quality continuously.
Alt Treatment is a free, independent platform that helps you understand stem cell therapy & decide if it’s right for you.
We break down complex information into clear, honest guidance. When you’re ready, we can connect you with verified clinics that meet your needs, in the right location, and often with exclusive discounts.
There’s no charge to use our platform. No hidden fees. No pressure. Our main aim is to genuinely help you figure out if treatment is right & the best places to consider.
If you want to talk, fill out our form here & our personal concierge team will reach out.
Japan, Colombia, South Korea, & Thailand are amongst the countries you can get Stem Cell treatments for back conditions in. To view clinics globally that can treat back conditions, you can compare them all here.
No, insurance companies won’t cover stem cell therapy for back pain because they still classify it as experimental.
In theory, some stem cells do have the potential to grow cancer tumors. Read more about it on our article covering Stem Cell treatment Side Effects.
Stem Cells can potentially help with most orthopedic conditions. Treatments are also available for autoimmune conditions, neurological issues etc.
Our guide on What Stem cells can help with might be useful.
Fill in your details below
For a discounted offer for Stem Cell Therapy!









